Fat Is Saturated Because It Contains All Single Bonds And Is Solid At Room Temperature

What fat is saturated because it contains all single bonds and is solid at room temperature.
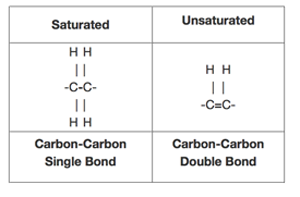
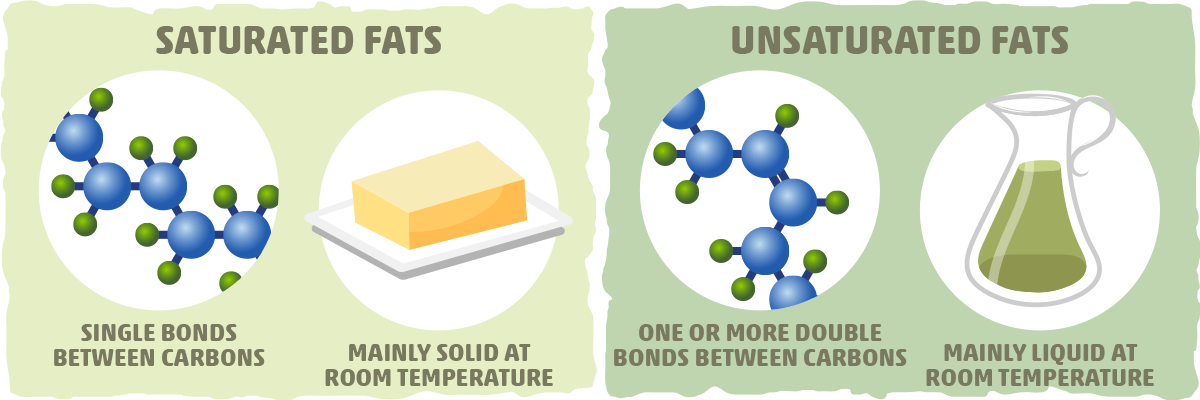
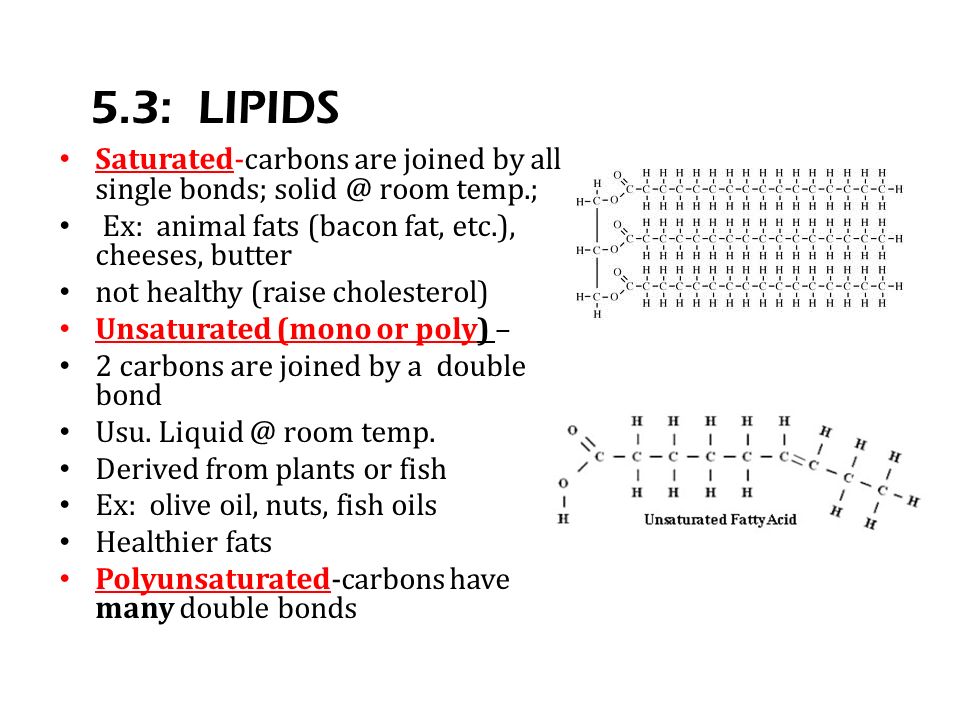
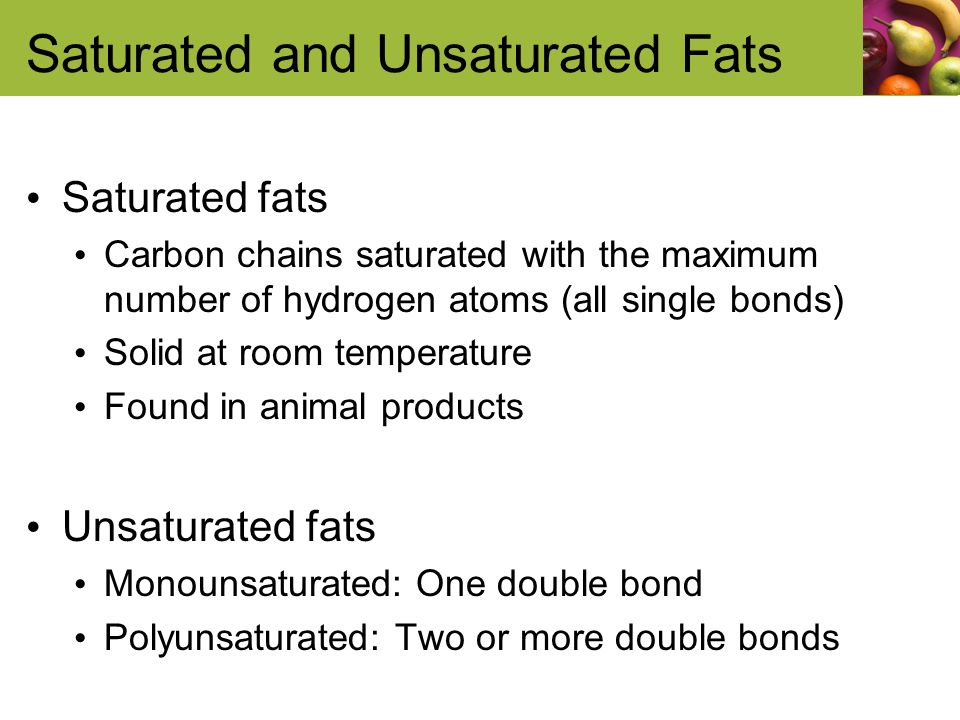
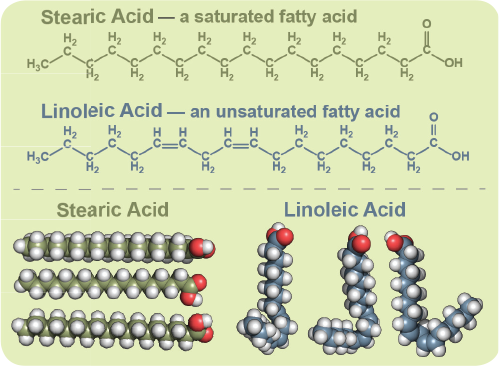
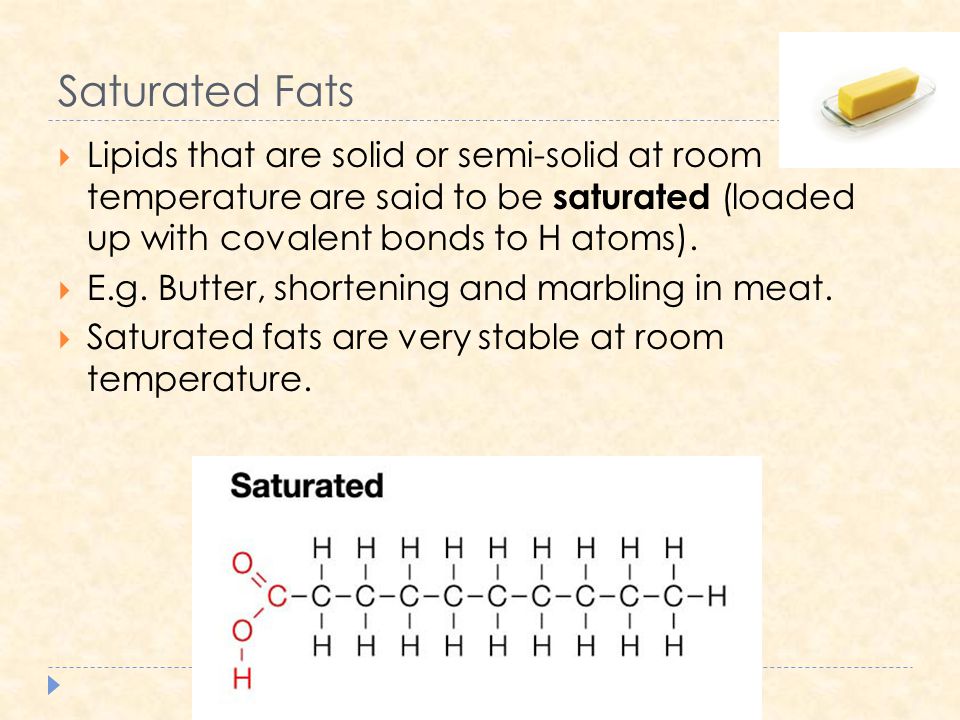
Fat is saturated because it contains all single bonds and is solid at room temperature. Butter margarine and animal fats are solid in the fridge. Double bonds can react with hydrogen to form single bonds. By contrast other fats have higher melting temperatures. Some carbon atoms are linked by single bonds c c and others are linked by double bonds c c.
There are some exceptions but most are solid at room temperature. The double bonding gives the molecules a. Most animal based food contains saturated fats. They are solid at room temperature and have a high melting point compared to unsaturated fats.
All bonds are single bonds in these fats. Glycerol and fatty acids fats are made of long chains of carbon c atoms. A saturated fat is a type of fat in which the fatty acid chains have all or predominantly single bonds a fat is made of two kinds of smaller molecules. Saturated fats are a type of fats that do not have double bonds between the molecules of fatty acid chains.
Some oils and fats are liquid at room temperature and even when kept in the fridge like olive oil and soybean oil. What are saturated fats. Sources of saturated fat.