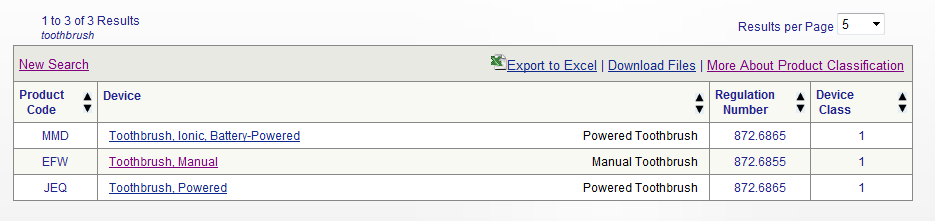
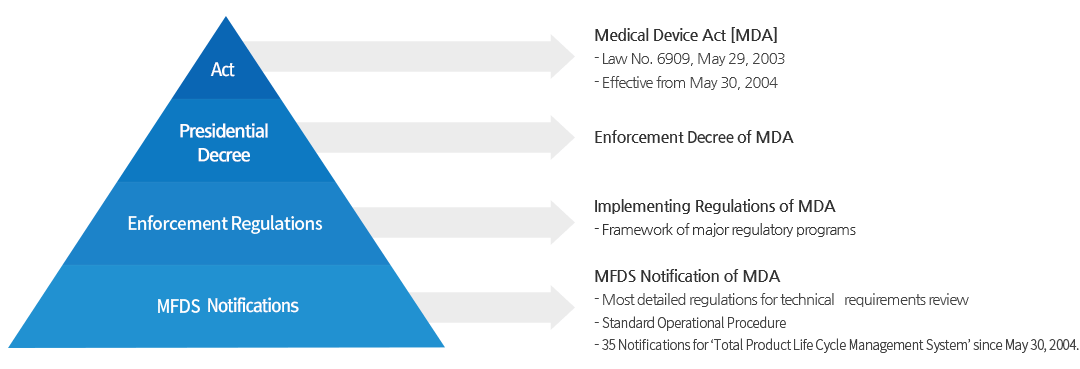
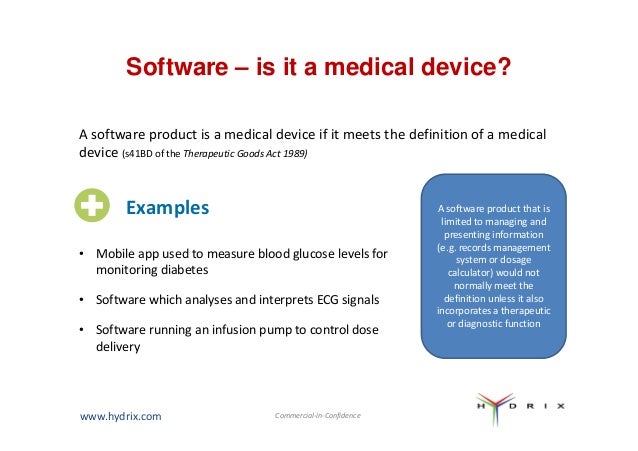
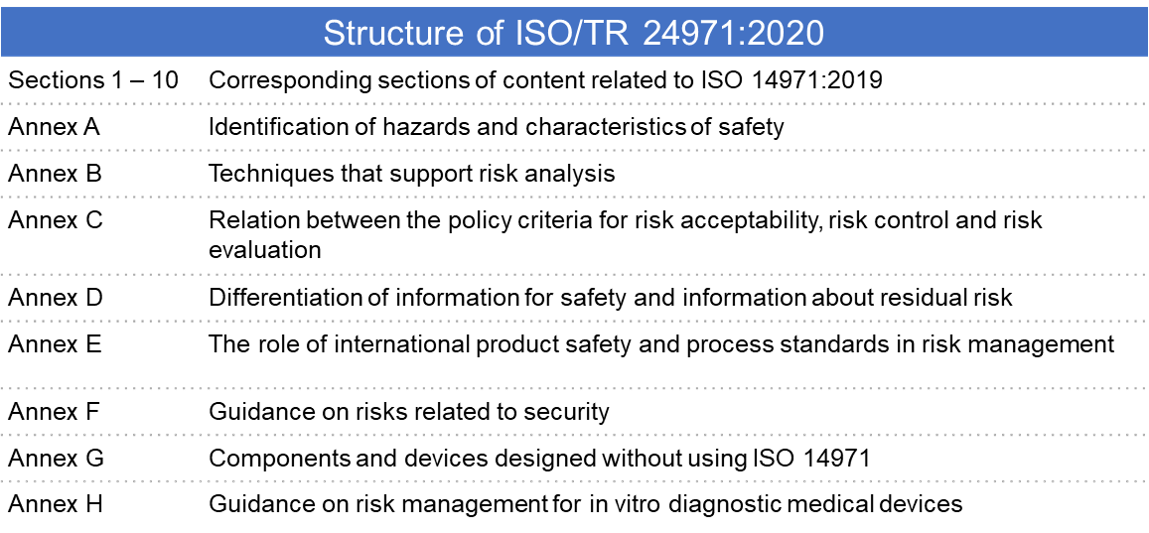
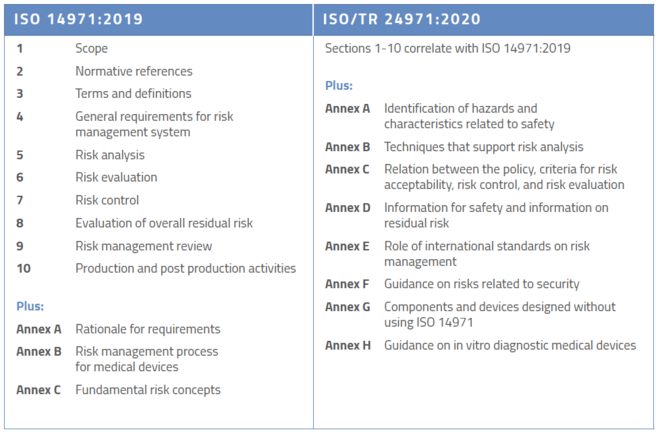
Diagnostic Medical Devices Definition

The term medical device as defined in the food and drugs act is any article instrument apparatus or contrivance including any component part or accessory thereof manufactured sold or represented for use in.
Diagnostic medical devices definition. In vitro diagnostics are tests done on samples such as blood or tissue that have been taken from the human body. The diagnosis treatment mitigation or prevention of a disease disorder or abnormal physical state or its symptoms in a human being. The medical devices and the in vitro diagnostic devices regulations have introduced new responsibilities for the european medicines agency ema and national competent authorities in the assessment of certain categories of medical device. The fda considers a product to be a device and subject to fda regulation if it meets the definition of a medical device per section 201 h of the food drug and cosmetic act.
The ivdd is implemented in the national laws of the member states. Importing fda medical device. A companion diagnostic device can be in vitro diagnostic device or an imaging tool that provides information that is essential for the safe and effective use of a corresponding therapeutic product. Medical devices are products or equipment intended generally for a medical use and are regulated at member state level.
In vitro diagnostics can detect diseases or other conditions and can be used to. Understanding the in vitro diagnostic medical devices directive 98 79 ec in vitro diagnostic medical devices ivds are subject to the european directive 98 79 ec ivdd. Definition of the terms medical device and in vitro diagnostic ivd medical device study group 1 final document ghtf sg1 n071 2012 may 16th 2012 page 6 of 6 5 0 definition of the terms medical device and in vitro diagnostic ivd medical device 5 1 medical device. Medical device full definition medical device means any instrument apparatus implement machine appliance implant reagent for in vitro use software material or other similar or related article intended by the manufacturer to be used alone or in combination for human beings for one or more of the specific medical purpose s of.